A) \[1.815\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
B) \[2.8\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
C) \[3.8\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
D) \[4.815\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
Correct Answer: A
Solution :
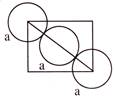 \[2({{r}_{C{{l}^{\Theta }}}}+{{r}_{C{{l}^{\Theta }}}})\,\,=\,\,\sqrt{2}\,a\] \[{{r}_{C{{l}^{\Theta }}}}+{{r}_{C{{l}^{\Theta }}}}\,=\,\frac{a}{\sqrt{2}}\,\,\Rightarrow \,\,{{r}_{C{{l}^{\Theta }}}}\,=\,\,\frac{a}{2\sqrt{2}}\,\]
\[2({{r}_{C{{l}^{\Theta }}}}+{{r}_{C{{l}^{\Theta }}}})\,\,=\,\,\sqrt{2}\,a\] \[{{r}_{C{{l}^{\Theta }}}}+{{r}_{C{{l}^{\Theta }}}}\,=\,\frac{a}{\sqrt{2}}\,\,\Rightarrow \,\,{{r}_{C{{l}^{\Theta }}}}\,=\,\,\frac{a}{2\sqrt{2}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
