A) \[{{\operatorname{BF}}^{-}}_{4}\]
B) \[{{\operatorname{NH}}^{+}}_{4}\]
C) \[{{\left[ \operatorname{Cu}{{\left( N{{H}_{3}} \right)}_{4}} \right]}^{2+}}\]
D) \[{{\operatorname{NiCl}}^{2-}}_{4}\]
Correct Answer: C
Solution :
\[{{\left[ \operatorname{Cu}{{\left( N{{H}_{3}} \right)}_{4}} \right]}^{2+}}\] It is square planar in shape.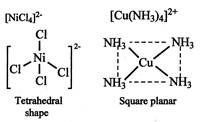
You need to login to perform this action.
You will be redirected in
3 sec
