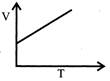
A)
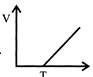
B)
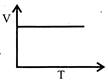
C)
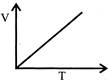
D)
Correct Answer: A
Solution :
\[{{\operatorname{V}}_{T}}={{V}_{o}}\left[ 1+\frac{T}{273} \right]\] \[{{\operatorname{V}}_{T}}={{V}_{o}}\frac{{{V}_{o}}T}{273}\] \[{{\operatorname{V}}_{T}}=\frac{{{V}_{o}}}{273}T+{{V}_{o}}\] Compare it with y=mx+c \[\operatorname{m}=\frac{{{V}_{o}}}{273}\,\,\,\,\,\,\,\,\,\,\,C={{V}_{o}}\]You need to login to perform this action.
You will be redirected in
3 sec
