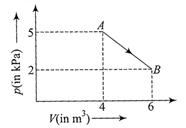
 The change in internal energy of the gas during the transition is
The change in internal energy of the gas during the transition is
A) 20 kJ
B) -20 kJ
C) 20 J
D) -12 kJ
Correct Answer: B
Solution :
The change in internal energy of the gas \[\Delta U=\frac{{{P}_{2}}{{V}_{2}}-{{P}_{1}}{{V}_{1}}}{\gamma -1}=\frac{2\times 6-4\times 5}{7}=-20\,kJ\]You need to login to perform this action.
You will be redirected in
3 sec
