A) \[3\,{{T}_{0}}\]
B) \[6\,{{T}_{0}}\]
C) \[4\,{{T}_{0}}\]
D) \[9\,{{T}_{0}}\]
Correct Answer: D
Solution :
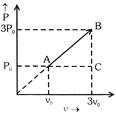
In process A to C (At constant pressure) So \[V\propto T\] \[\frac{{{T}_{C}}}{{{T}_{A}}}=\frac{3{{V}_{0}}}{{{V}_{0}}}\] \[{{T}_{C}}=3{{T}_{0}}\] In process C to B (At constant volume) \[P\propto T\] \[\frac{{{T}_{B}}}{{{T}_{C}}}=\frac{3{{P}_{0}}}{{{P}_{0}}}\] \[{{T}_{B}}=3{{T}_{C}}\] so \[{{T}_{B}}=3\times 3{{T}_{0}}=9{{T}_{0}}\]You need to login to perform this action.
You will be redirected in
3 sec
