A) \[4\text{ }Cr-O\]bonds are equivalent
B) \[\text{6 }Cr-O\] bonds are equivalent
C) all \[Cr-O\] bonds are equivalent
D) all \[Cr-O\] bonds are non-equivalent
Correct Answer: B
Solution :
The structure of dichromate ion is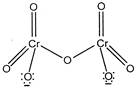 \[C{{r}_{2}}O_{7}^{2-}\] exhibit resonance phenomena. Except the bridged \[Cr-O-Cr,\]all the \[Cr-O\]bonds are equivalent.
\[C{{r}_{2}}O_{7}^{2-}\] exhibit resonance phenomena. Except the bridged \[Cr-O-Cr,\]all the \[Cr-O\]bonds are equivalent.
You need to login to perform this action.
You will be redirected in
3 sec
