A)
![]()
B)
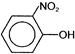
C)

D)
![]()
Correct Answer: D
Solution :
OH group donates electrons while \[N{{O}_{2}}\] group accepts electrons. As a result, centres of positive and negative charge are created. Since dipole moment is the product of charge and distance. Hence, p-nitrophenol in which positive and negative charges are separated by maximum distance has the highest dipole moment.You need to login to perform this action.
You will be redirected in
3 sec
