A) 30 K
B) 18 K
C) 50 K
D) 42 K
Correct Answer: D
Solution :
F-B-D of piston of cylinder A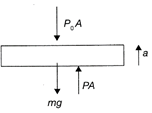 Where,\[{{P}_{0}}\]is the atmospheric pressure, mg the weight of the piston, a the acceleration of the piston and P, the pressure of the gas. Invoking Newton's Second law, \[F=ma,\]we get: \[PA-{{P}_{0}}A-mg=ma\] Assuming slow movement, the acceleration of the piston, i.e., \[a=0,\]we get: \[PA-{{P}_{0}}A-mg=0\Rightarrow P=\frac{mg+{{P}_{0}}A}{A}=\]constant So, for A, the process is Isobaric. \[\therefore \]\[\Delta Q=\mu {{C}_{p}}(\Delta \Tau )\,t\] For B the process is Isochoric, \[\therefore \]\[\Delta Q=\mu C,{{(\Delta \Tau )}_{2}}\] Now, \[{{C}_{p}}{{(\Delta T)}_{1}}={{C}_{v}}{{(\Delta T)}_{2}}\]or \[\frac{7R}{2}\times 30=\frac{5R}{2}{{(\Delta \Tau )}_{2}}\] \[\Delta {{\Tau }_{2}}=42\,K\] Hence, the correction option is [d].
Where,\[{{P}_{0}}\]is the atmospheric pressure, mg the weight of the piston, a the acceleration of the piston and P, the pressure of the gas. Invoking Newton's Second law, \[F=ma,\]we get: \[PA-{{P}_{0}}A-mg=ma\] Assuming slow movement, the acceleration of the piston, i.e., \[a=0,\]we get: \[PA-{{P}_{0}}A-mg=0\Rightarrow P=\frac{mg+{{P}_{0}}A}{A}=\]constant So, for A, the process is Isobaric. \[\therefore \]\[\Delta Q=\mu {{C}_{p}}(\Delta \Tau )\,t\] For B the process is Isochoric, \[\therefore \]\[\Delta Q=\mu C,{{(\Delta \Tau )}_{2}}\] Now, \[{{C}_{p}}{{(\Delta T)}_{1}}={{C}_{v}}{{(\Delta T)}_{2}}\]or \[\frac{7R}{2}\times 30=\frac{5R}{2}{{(\Delta \Tau )}_{2}}\] \[\Delta {{\Tau }_{2}}=42\,K\] Hence, the correction option is [d].
You need to login to perform this action.
You will be redirected in
3 sec
