A) 0.6 N
B) 0.1 N
C) 0.3 N
D) 0.9 N
Correct Answer: D
Solution :
\[\text{Normality = Molarity }\!\!\times\!\!\text{ n}\] \[n=\]Number of replaceable H atom or OH groups in a molecule. In \[{{H}_{3}}P{{O}_{4}}\](orthophosphoric acid) the value of n=3.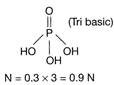 Hence, the correct option is [d].
Hence, the correct option is [d].
You need to login to perform this action.
You will be redirected in
3 sec
