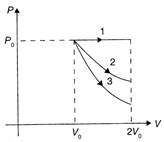
A) \[\Delta {{U}_{1}}>\Delta {{U}_{2}}>\Delta {{U}_{3}}\]
B) \[\Delta {{U}_{1}}<\Delta {{U}_{2}}<\Delta {{U}_{3}}\]
C) \[\Delta {{U}_{2}}<\Delta {{U}_{1}}<\Delta {{U}_{3}}\]
D) \[\Delta {{U}_{2}}<\Delta {{U}_{3}}<\Delta {{U}_{1}}\]
Correct Answer: A
Solution :
For all processes, \[\Delta U=n{{c}_{v}}\Delta T\] For isobaric process \[\Delta {{U}_{1}}=n{{c}_{v}}\Delta T\] In isobaric expansion \[\Delta T\]is positive, therefore \[\Delta {{U}_{1}}=\]positive For isothermal process\[\Delta {{U}_{2}}=n{{c}_{v}}\Delta T=0\] For adiabatic process \[\Delta {{U}_{3}}=n{{c}_{v}}\Delta T\] In adiabatic expansion, the temperature decreases, therefore \[\Delta {{U}_{3}}\]is negative \[\therefore \]\[\Delta {{U}_{1}}>\Delta {{U}_{2}}>\Delta {{U}_{3}}.\] Hence, the correction option is [a].You need to login to perform this action.
You will be redirected in
3 sec
