A) p-p orbitals
B) s-s orbitals
C) s-p orbitals
D) s-d orbitals
Correct Answer: A
Solution :
In the formation of pi bond the atomic orbitals overlap in such a way that their axes remain parallel to each other and perpendicular to the internuclear axis. The orbitals formed due to sidewise overlapping consists of two saucer type charged clouds above and below the plane of the participating atoms.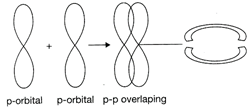 Hence, the correct option is [a].
Hence, the correct option is [a].
You need to login to perform this action.
You will be redirected in
3 sec
