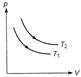
A) \[{{T}_{1}}>{{T}_{2}}\]
B) \[{{T}_{1}}<{{T}_{2}}\]
C) \[{{T}_{1}}={{T}_{2}}\]
D) Cannot be predicted
Correct Answer: B
Solution :
Because p-V curve at \[{{T}_{2}}\] is above than at \[{{T}_{1}}\] and \[pV=nRT,\] so for same number of moles \[{{T}_{2}}>{{T}_{1}}\]You need to login to perform this action.
You will be redirected in
3 sec
