| (I) \[C{{H}_{3}}-{{\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{{{C}^{\oplus }}}}}\,}^{{}}}\] | (II) 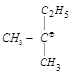 |
| (III)\[C{{H}_{3}}-{{\underset{\begin{smallmatrix} | \\ {{C}_{2}}{{H}_{5}} \end{smallmatrix}}{\overset{\begin{smallmatrix} {{C}_{2}}{{H}_{5}} \\ | \end{smallmatrix}}{\mathop{{{C}^{\oplus }}}}}\,}^{{}}}\] | (IV)\[{{C}_{2}}{{H}_{5}}\,-{{\underset{\begin{smallmatrix} | \\ {{C}_{2}}{{H}_{5}} \end{smallmatrix}}{\overset{\begin{smallmatrix} {{C}_{2}}{{H}_{5}} \\ | \end{smallmatrix}}{\mathop{{{C}^{\oplus }}}}}\,}^{{}}}\] |
A) IV > III > II > I
B) IV > III > I > II
C) I > II > III > IV
D) I > III > II > IV
Correct Answer: C
Solution :
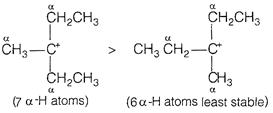
The stability is determined by hyperconjugation. More the number of a-hydrpgen atoms, more is the stability.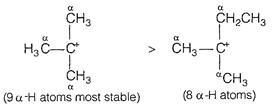
You need to login to perform this action.
You will be redirected in
3 sec
