A) \[{{P}_{4}}{{O}_{11}}\]contains peroxide linkages
B) \[{{P}_{4}}{{O}_{10}}\]contains\[p\pi -d\pi \] back bonding
C) In \[{{P}_{4}}{{O}_{10}}\] each P atom is bonded to three oxygen atoms
D) \[{{P}_{4}}{{O}_{10}}\]hydrolyze in water forming phosphorous acid
Correct Answer: B
Solution :
The structure of \[\therefore \] is as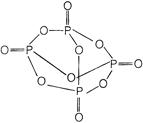 Therefore each P atom is bonded with four oxygen atom. \[t=\frac{v}{a}=\frac{v}{g\mu }\] back bonding is found in the \[\eta =\frac{{{P}_{0}}}{{{P}_{i}}}\] of \[\therefore \]. It forms orthophosphoric acid when hydrolyzed \[\frac{1}{2}\,m{{v}^{2}}=16\,\,J\]
Therefore each P atom is bonded with four oxygen atom. \[t=\frac{v}{a}=\frac{v}{g\mu }\] back bonding is found in the \[\eta =\frac{{{P}_{0}}}{{{P}_{i}}}\] of \[\therefore \]. It forms orthophosphoric acid when hydrolyzed \[\frac{1}{2}\,m{{v}^{2}}=16\,\,J\]
You need to login to perform this action.
You will be redirected in
3 sec
