| Directions: The decarboxylation of aromatic acids is most often carried out by heating with Cu-quinoline \[ArCOOH\xrightarrow[{}]{Cu-quinoline}ArH+C{{O}_{2}}\] Cuprous salts of aromatic acids, actually undergoes decarboxylation. However, two other methods can be used with certain substrates. |
| Method 1: Salt of acid, \[ArCO{{O}^{-}}\]is heated (SE1) |
Step I: 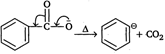 |
| Step II: |
Method II: Carboxylic acid is heated with a strong acid, often sulphuric acid. 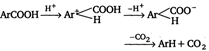 |
Decarboxylation takes place by the arenium ion mechanism, with\[{{\text{H}}^{+}}\] electrophile. Evidently, the order of electrofugal ability is \[C{{O}_{2}}>{{H}^{+}}>COO{{H}^{+}}\]Rearrangements are also known to take place. For example,, when the phthalate ion is heated with catalytic amount of cadmium, the terephthalate ion is produced. 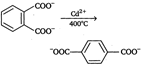 In a similar process, potassium benzoate heated with cadmium salts disproportionates. The rearrangement is named as 'Henkel rearrangement'. In a similar process, potassium benzoate heated with cadmium salts disproportionates. The rearrangement is named as 'Henkel rearrangement'. |
A) \[-OC{{H}_{3}}>-N{{O}_{2}}>-F>-H>-C{{H}_{3}}\]
B) \[-N{{O}_{2}}>-F>-H>-C{{H}_{3}}>-OC{{H}_{3}}\]
C) \[-N{{O}_{2}}>-F>-OC{{H}_{3}}>-H>-C{{H}_{3}}\]
D) \[-OC{{H}_{3}}>-C{{H}_{3}}>-H>-F>-N{{O}_{2}}\]
Correct Answer: C
Solution :
You need to login to perform this action.
You will be redirected in
3 sec
