A) 1
B) 2
C) 3
D) 4
Correct Answer: C
Solution :
\[\frac{80.6}{100}\times 15=\Delta E\] \[=12.09\] \[=|{{E}_{3}}-{{E}_{1}}|\]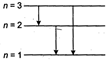 So, \[{{e}^{-1}}\]can make the hydrogen atom to go into 2nd exited state ie, n = 3 energy state. From this 3 transitions are possible ie, from 3 to 2, 3 to 1 and 2 to 1.
So, \[{{e}^{-1}}\]can make the hydrogen atom to go into 2nd exited state ie, n = 3 energy state. From this 3 transitions are possible ie, from 3 to 2, 3 to 1 and 2 to 1.
You need to login to perform this action.
You will be redirected in
3 sec
