| Directions: Based on following paragraph. |
| In certain polar solvents, \[\text{PC}{{\text{l}}_{\text{s}}}\]undergoes an ionization reaction in which \[\text{C}{{\text{l}}^{-}}\]ion leaves one \[\text{PC}{{\text{l}}_{\text{5}}}\]molecule and attaches itself to another. \[\text{2PC}{{\text{l}}_{\text{5}}}\text{PCl}_{\text{4}}^{\text{+}}\text{+PCl}_{\text{6}}^{\text{-}}\] |
A) 0, 1, 2
B) 0, 0, 0
C) 1, 2, 3
D) 0, 2, 1
Correct Answer: B
Solution :
\[PC{{l}_{5}}\Rightarrow \]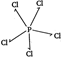 (no lone pair of electron)
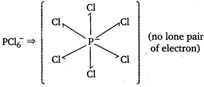
(no lone pair of electron) 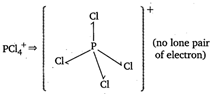
You need to login to perform this action.
You will be redirected in
3 sec
