A) \[\text{2}\text{.11 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-34}}}\text{J-s}\]
B) \[\text{3}\text{.16 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-34}}}\text{J-s}\]
C) \[\text{1}\text{.05 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-34}}}\text{J-s}\]
D) \[\text{4}\text{.22 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{-34}}}\text{J-s}\]
Correct Answer: C
Solution :
When 91.8 eV energy has been absorbed, electron transits in n = 2 state.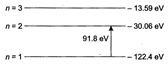 Change in angular momentum \[=\frac{2h}{2\pi }-\frac{h}{2\pi }\] \[=\frac{h}{2\pi }\] \[=1.05\times {{10}^{-34}}\text{J-s}\]
Change in angular momentum \[=\frac{2h}{2\pi }-\frac{h}{2\pi }\] \[=\frac{h}{2\pi }\] \[=1.05\times {{10}^{-34}}\text{J-s}\]
You need to login to perform this action.
You will be redirected in
3 sec
