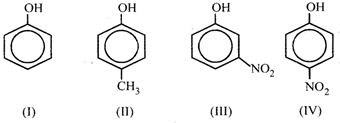
 the order of acidity is
the order of acidity is
A) III > IV > I > II
B) I > IV >III > II
C) II > I > III > IV
D) IV > III > I < II
Correct Answer: D
Solution :
-NO, group is electron withdrawing, and hence III and IV are strong acids whereas \[-C{{H}_{3}}\] is electron releasing group, hence I is less acidic than the others.You need to login to perform this action.
You will be redirected in
3 sec
