A) \[O{{F}_{2}}<Sn{{F}_{2}}<B{{F}_{3}}\]
B) \[Sn{{F}_{2}}<O{{F}_{2}}<B{{F}_{3}}\]
C) \[O{{F}_{2}}<B{{F}_{3}}<Sn{{F}_{2}}\]
D) \[B{{F}_{2}}<Sn{{F}_{2}}<O{{F}_{2}}\]
Correct Answer: A
Solution :
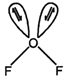 \[H.B.\,=2\sigma +2\] long pair = 4 \[\Rightarrow \,\] V-shape \[(s{{p}^{3}})\Rightarrow \,{{105}^{0}}\]
\[H.B.\,=2\sigma +2\] long pair = 4 \[\Rightarrow \,\] V-shape \[(s{{p}^{3}})\Rightarrow \,{{105}^{0}}\] 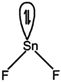 \[H.B=2\sigma +1\] lone pair \[=3(s{{p}^{2}})\] \[\Rightarrow \] Bent shape \[=\text{ }{{118}^{o}}\]
\[H.B=2\sigma +1\] lone pair \[=3(s{{p}^{2}})\] \[\Rightarrow \] Bent shape \[=\text{ }{{118}^{o}}\] 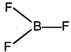 \[H.B=3\sigma +0\] lone pair\[=3(s{{p}^{2}})\] \[\Rightarrow \] Trigonal planar \[=\text{ }{{120}^{o}}\]
\[H.B=3\sigma +0\] lone pair\[=3(s{{p}^{2}})\] \[\Rightarrow \] Trigonal planar \[=\text{ }{{120}^{o}}\]
You need to login to perform this action.
You will be redirected in
3 sec
