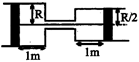
A) \[\frac{4}{5}{{P}_{0}}\]
B) \[\frac{3}{5}{{P}_{0}}\]
C) \[\frac{2}{5}{{P}_{0}}\]
D) \[\frac{5}{4}{{P}_{0}}\]
Correct Answer: D
Solution :
Initial volume \[=1\,\times \left[ \pi {{R}^{2}}\,+\frac{\pi {{R}^{2}}}{4} \right]=\frac{5}{4}\pi {{R}^{2}}\] Piston moves towards left by one metre on heating. Additional volume available due to heating \[=1\times \pi {{R}^{2}}-\frac{1+\pi {{R}^{2}}}{4}=\frac{3}{4}\,\pi {{R}^{2}}\] Total volume after heating \[=2\pi {{R}^{2}}\] \[{{P}_{0}}\times \frac{5}{4}\,\pi {{R}^{2}}\,=nR{{T}_{0}}\] ?(1) After heating, \[{{P}_{2}}\,\times 2\pi {{R}^{2}}\,=nR\,\times 2{{T}_{0}}\] ...(2) \[\frac{{{P}_{0}}}{{{P}_{2}}}\times \frac{5}{4}\times \frac{1}{2}\,=\frac{1}{2}\] \[{{P}_{2}}=\frac{5}{4}\,{{P}_{0}}\]You need to login to perform this action.
You will be redirected in
3 sec
