A)
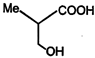
B)
![]()
C)
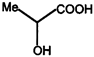
D)
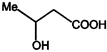
Correct Answer: A
Solution :
\[NaHC{{O}_{3}}\]test shows the presence of the (-COOH) group, and from the structures given in the problem, only the compound in (A) on reduction with \[LiAl{{H}_{4}}\] gives a chiral product.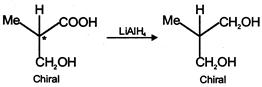 The compounds (2), (3) and (4) with \[LiAl\,{{H}_{4}}\] will give chiral products. So the answer is (1)
The compounds (2), (3) and (4) with \[LiAl\,{{H}_{4}}\] will give chiral products. So the answer is (1)
You need to login to perform this action.
You will be redirected in
3 sec
