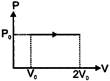
A) \[{{P}_{0}}{{V}_{0}}\]
B) \[\frac{{{P}_{0}}{{V}_{0}}}{2}\]
C) \[3{{P}_{0}}{{V}_{0}}\]
D) \[\frac{3}{2}{{P}_{0}}{{V}_{0}}\]
Correct Answer: A
Solution :
According to first law of thermodynamics \[du=dq-dw\] \[dw=-\int{{{P}_{ext}}\cdot dv=-{{P}_{0}}{{V}_{0}}}\] \[\therefore dq=2{{P}_{0}}{{V}_{0}}-{{P}_{0}}{{V}_{0}}\] \[dq={{P}_{0}}{{V}_{0}}\] Heat absorbed in the process will be\[{{P}_{0}}{{V}_{0}}\].You need to login to perform this action.
You will be redirected in
3 sec
