A) All \[s{{p}^{2}}\] hybridised
B) Three \[s{{p}^{2}}\] and one \[s{{p}^{3}}\] hybridised
C) One \[s{{p}^{2}}\] and three \[s{{p}^{3}}\] hybridised
D) Two \[s{{p}^{2}}\] and two \[s{{p}^{3}}\] hybridized
Correct Answer: D
Solution :
Borax \[(N{{a}_{2}}{{B}_{4}}{{O}_{7}}.10\,\,{{H}_{2}}O)\]is actually made from two tetrahedral and two triangular units joined as shown in figure and should be written as\[N{{a}_{2}}({{B}_{4}}{{O}_{5}}{{(OH)}_{4}}.8{{H}_{2}}O)\].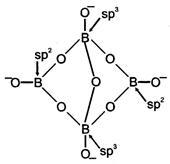
You need to login to perform this action.
You will be redirected in
3 sec
