A)
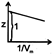
B)
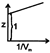
C)
![]()
D)
![]()
Correct Answer: A
Solution :
At low pressure vander waal's equation for a real gas is given as \[Z=1-\frac{a}{RTV}\] intercept = 1 slop = -veYou need to login to perform this action.
You will be redirected in
3 sec
