A) Boric acid is a protonic acid.
B) Both \[T{{i}^{3+}}\] and \[A{{l}^{3+}}\] ions act as oxidsing agent in aqueous solution.
C) Hydrogen bonding in \[{{H}_{3}}B{{O}_{3}}\]gives it a layered structure.
D) Aqueous solution of borax is acidic
Correct Answer: C
Solution :
In the solid state, the \[B{{(OH)}_{3}}\] units are hydrogen bonded together in to two dimensional sheets with almost hexagonal symmetry.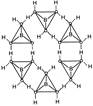
You need to login to perform this action.
You will be redirected in
3 sec
