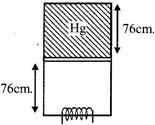
| Statement 1: The temperature of gas continuously increases. |
| Statement 2: According to first law of thermodynamics \[dQ=dU+dW\] where symbols have their usual meaning. |
A) Statement-1 is false, Statement-2 is true.
B) Statement-1 is true, Statement-2 is true; Statement-2 is a correct explanation for Statement-1
C) Statement-1 is true, Statement-2 is true; Statement-2 is not a correct explanation for Statement-1
D) Statement-1 is true, Statement-2 is false.
Correct Answer: A
Solution :
Initial condition Volume \[=76cm\times A\] Pressure \[=(76+76)dg\,=152\,dg\] Final condition Volume \[=152cm\times A\] Pressure \[=76\,dg\] \[\therefore \] \[{{O}_{1}}{{V}_{1}}={{P}_{2}}{{V}_{2}}\] \[\therefore \] Both points lie on the same isothermal line i.e. both have same T.You need to login to perform this action.
You will be redirected in
3 sec
