A) 44.8 mL
B) 73.1 mL
C) 123.01 mL
D) 0
Correct Answer: D
Solution :
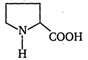 It consists of \[2{}^\circ \] amino group that's why diazotisation does not proceed, resultantly no evolution of \[{{N}_{2}}\]. It is not proper for van Slyke method.
It consists of \[2{}^\circ \] amino group that's why diazotisation does not proceed, resultantly no evolution of \[{{N}_{2}}\]. It is not proper for van Slyke method.
You need to login to perform this action.
You will be redirected in
3 sec
