A)
![]()
B)
![]()
C)
![]()
D)
![]()
Correct Answer: C
Solution :
Benzene is non-polar in nature. As we know that non-polar disperses more to non-polar substances. Therefore, meta-metyl nonylbenzene being non- polar from both sides will disperse more to benzene. All other substances (a, b and d) have either one side polar or both sides polar.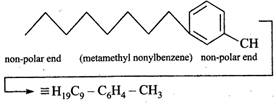
You need to login to perform this action.
You will be redirected in
3 sec
