A) Alkaline\[{{H}_{2}}{{O}_{2}}\], acidified\[{{H}_{2}}{{O}_{2}}\], on standing
B) Alkaline\[{{O}_{3}}\], acidified\[{{O}_{3}}\],\[Zn/HCl\]
C) Acidified\[{{H}_{2}}{{O}_{2}}\], alkaline\[{{H}_{2}}{{O}_{2}}\], heat
D) Alkaline\[{{O}_{3}}\], heat, \[N{{H}_{4}}OH\]
Correct Answer: A
Solution :
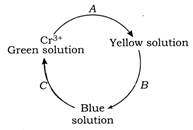
[A] \[\underset{\text{Green}}{\mathop{2l{{r}^{3+}}}}\,+10O{{H}^{-}}+3{{H}_{2}}{{O}_{2}}\xrightarrow{{}}\underset{\text{Yellow}}{\mathop{2CrO_{4}^{2-}}}\,+{{H}_{2}}O\] [B] \[Cr_{4}^{2-}+2{{H}_{2}}{{O}_{2}}+2{{H}^{+}}\xrightarrow{{}}\]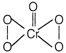 \[+3{{H}_{2}}O\] In aqueous solution, 005 is unstable and it further decomposes. [C] \[2Cr{{O}_{5}}\xrightarrow{{}}\underset{\left( \text{Amphoteric} \right)}{\mathop{C{{r}_{2}}{{O}_{3}}+\frac{7}{2}{{O}_{2}}}}\,\] \[C{{r}_{2}}{{O}_{3}}+3{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}C{{r}_{2}}{{(S{{O}_{4}})}_{3}}+3{{H}_{2}}O\]
\[+3{{H}_{2}}O\] In aqueous solution, 005 is unstable and it further decomposes. [C] \[2Cr{{O}_{5}}\xrightarrow{{}}\underset{\left( \text{Amphoteric} \right)}{\mathop{C{{r}_{2}}{{O}_{3}}+\frac{7}{2}{{O}_{2}}}}\,\] \[C{{r}_{2}}{{O}_{3}}+3{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}C{{r}_{2}}{{(S{{O}_{4}})}_{3}}+3{{H}_{2}}O\]
You need to login to perform this action.
You will be redirected in
3 sec
