| (I) |
(II) 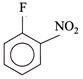 |
(III) 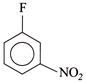 |
(IV) |
A) \[I>III>IV>II\]
B) \[II>IV>III>I\]
C) \[II>IV>I>III\]
D) \[IV>II>III>I\]
Correct Answer: B
Solution :
[b] \[II>IV>III>I\] The leaving group F is same, so the ArSN reactivity is determined by EWG \[(-N{{O}_{2}})\] group. More the EWG, more rapid is the reaction. EW power of \[(-N{{O}_{2}})\] at \[o>p>m\]. Although negative charge in aren anion, is equally stabilised by \[(-N{{O}_{2}})\] group at o- and p-position, -I power of \[(-N{{O}_{2}})\] group is slightly more at ortho than at para.You need to login to perform this action.
You will be redirected in
3 sec
