A) \[\frac{{{n}^{2}}{{\hbar }^{2}}}{2({{m}_{1}}+{{m}_{2}}){{r}^{2}}}\]
B) \[\frac{2{{n}^{2}}{{\hbar }^{2}}}{({{m}_{1}}+{{m}_{2}}){{r}^{2}}}\]
C) \[\frac{({{m}_{1}}+{{m}_{2}}){{n}^{2}}{{\hbar }^{2}}}{2{{m}_{1}}{{m}_{2}}{{r}^{2}}}\]
D) \[\frac{{{({{m}_{1}}+{{m}_{2}})}^{2}}{{n}^{2}}{{\hbar }^{2}}}{2m_{1}^{2}m_{2}^{2}{{r}^{2}}}\]
Correct Answer: C
Solution :
[c] : A diatomic molecule consists of two atoms of masses \[{{m}_{1}}\]and \[{{m}_{2}}\]at a distance r apart. Let \[{{r}_{1}}\]and\[{{r}_{2}}\]be the distances of the atoms from the centre of mass.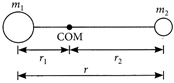 The moment of inertia of this molecule about an axis passing through its centre of mass and perpendicular to a line joining the atoms is \[I={{m}_{1}}r_{1}^{2}+{{m}_{2}}r_{2}^{2}\] As\[{{m}_{1}}{{r}_{1}}={{m}_{2}}{{r}_{2}}\]or\[{{r}_{1}}=\frac{{{m}_{2}}}{{{m}_{1}}}{{r}_{2}}\] \[\therefore \]\[{{r}_{1}}=\frac{{{m}_{2}}}{{{m}_{1}}}(r-{{r}_{1}});{{r}_{1}}=\frac{{{m}_{2}}r}{{{m}_{1}}+{{m}_{2}}}\] Similarly,\[{{r}_{2}}=\frac{{{m}_{1}}r}{{{m}_{1}}+{{m}_{2}}}\] Therefore, the moment of inertia can be written as \[I={{m}_{1}}{{\left( \frac{{{m}_{2}}r}{{{m}_{1}}+{{m}_{2}}} \right)}^{2}}+{{m}_{2}}{{\left( \frac{{{m}_{2}}r}{{{m}_{1}}+{{m}_{2}}} \right)}^{2}}\] \[=\frac{{{m}_{1}}{{m}_{2}}}{{{m}_{1}}+{{m}_{2}}}{{r}^{2}}\] ...(i) According to Bohr's quantization condition \[L=\frac{nh}{2\pi }\]or\[{{L}^{2}}=\frac{{{n}^{2}}{{h}^{2}}}{4{{\pi }^{2}}}\] ...(ii) Rotational energy, \[E=\frac{{{L}^{2}}}{2I}\] \[E=\frac{{{n}^{2}}{{h}^{2}}}{8{{\pi }^{2}}I}=\frac{{{n}^{2}}{{\hbar }^{2}}({{m}_{1}}+{{m}_{2}})}{2{{m}_{1}}{{m}_{2}}{{r}^{2}}}\](Using (i) and (ii))
The moment of inertia of this molecule about an axis passing through its centre of mass and perpendicular to a line joining the atoms is \[I={{m}_{1}}r_{1}^{2}+{{m}_{2}}r_{2}^{2}\] As\[{{m}_{1}}{{r}_{1}}={{m}_{2}}{{r}_{2}}\]or\[{{r}_{1}}=\frac{{{m}_{2}}}{{{m}_{1}}}{{r}_{2}}\] \[\therefore \]\[{{r}_{1}}=\frac{{{m}_{2}}}{{{m}_{1}}}(r-{{r}_{1}});{{r}_{1}}=\frac{{{m}_{2}}r}{{{m}_{1}}+{{m}_{2}}}\] Similarly,\[{{r}_{2}}=\frac{{{m}_{1}}r}{{{m}_{1}}+{{m}_{2}}}\] Therefore, the moment of inertia can be written as \[I={{m}_{1}}{{\left( \frac{{{m}_{2}}r}{{{m}_{1}}+{{m}_{2}}} \right)}^{2}}+{{m}_{2}}{{\left( \frac{{{m}_{2}}r}{{{m}_{1}}+{{m}_{2}}} \right)}^{2}}\] \[=\frac{{{m}_{1}}{{m}_{2}}}{{{m}_{1}}+{{m}_{2}}}{{r}^{2}}\] ...(i) According to Bohr's quantization condition \[L=\frac{nh}{2\pi }\]or\[{{L}^{2}}=\frac{{{n}^{2}}{{h}^{2}}}{4{{\pi }^{2}}}\] ...(ii) Rotational energy, \[E=\frac{{{L}^{2}}}{2I}\] \[E=\frac{{{n}^{2}}{{h}^{2}}}{8{{\pi }^{2}}I}=\frac{{{n}^{2}}{{\hbar }^{2}}({{m}_{1}}+{{m}_{2}})}{2{{m}_{1}}{{m}_{2}}{{r}^{2}}}\](Using (i) and (ii))
You need to login to perform this action.
You will be redirected in
3 sec
