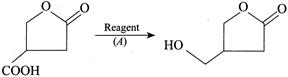
 The reagent is
The reagent is
A) LAH
B) \[HI+P\]
C) \[NaAl{{H}_{4}}\]
D) \[{{B}_{2}}{{H}_{6}}/{{H}_{2}}O\]
Correct Answer: D
Solution :
[d] 1. LAH reduces both ester and acid to alcohols.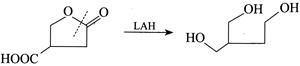 2. \[HI+P\]reduces both ester and acid to alkane.
2. \[HI+P\]reduces both ester and acid to alkane. 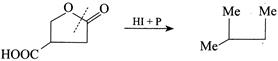 3. \[NaAl{{H}_{4}}\] reduces only ester to aldehyde and alcohol.
3. \[NaAl{{H}_{4}}\] reduces only ester to aldehyde and alcohol.  4. \[{{B}_{2}}{{H}_{6}}/{{H}_{2}}O\] selectively reduces acid to alcohol (although it can also reduce ester to alcohols) but acid group is reduced in the presence of ester.
4. \[{{B}_{2}}{{H}_{6}}/{{H}_{2}}O\] selectively reduces acid to alcohol (although it can also reduce ester to alcohols) but acid group is reduced in the presence of ester. 
You need to login to perform this action.
You will be redirected in
3 sec
