A) \[\sqrt{3/2}\]
B) \[\sqrt{2}\]
C) \[\sqrt{2/3}\]
D) \[\sqrt{3}\]
Correct Answer: B
Solution :
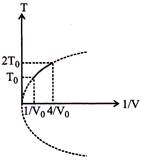
| From graph, \[{{T}^{2}}V\]= const. | .....(1) |
| As we know that \[T{{V}^{\gamma -1}}=\] const. | |
| \[\Rightarrow \,\,\,\,V{{T}^{\frac{1}{\gamma -1}}}=\] cons. | ....(2) |
| On comparing (1) and (2), we get | |
| \[\Rightarrow \,\,\,\gamma =3/2\] | |
| Also \[{{\text{v}}_{rms}}=\sqrt{\frac{3P}{\rho }}\] and \[{{\text{v}}_{sound}}=\sqrt{\frac{P\gamma }{\rho }}\] | |
| \[\Rightarrow \,\,\,\frac{{{\text{v}}_{rms}}}{{{\text{v}}_{sound}}}=\sqrt{\frac{3}{\gamma }}=\sqrt{2}\] | |
You need to login to perform this action.
You will be redirected in
3 sec
