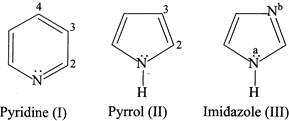
 Which statement is INCORRECT?
Which statement is INCORRECT?
A) and (III) are modest Bronsted base whereas (II) is not.
B) In (III) \[{{N}^{a}}\] is more basic than\[{{N}^{b}}\].
C) When (II) is protonated in the presence of a strong acid, protonation occurs at C-2.
D) All the nitrogen present in (I), (II), and (III) are \[s{{p}^{2}}\]hybridised.
Correct Answer: B
Solution :
[b] (I)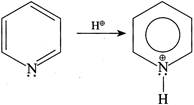 (III)
(III) 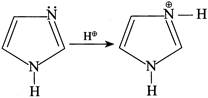 But (II) is not protonated, hence the statement (I) is true. In (II):
But (II) is not protonated, hence the statement (I) is true. In (II):  So, \[{{N}^{b}}\] is more basic due to the presence of LP \[\bar{e}'s\]. Hence, the statement (2) is wrong Statement (3):
So, \[{{N}^{b}}\] is more basic due to the presence of LP \[\bar{e}'s\]. Hence, the statement (2) is wrong Statement (3): 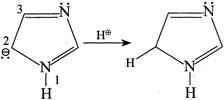 Statement (3) is true. Statement (4): Due to resonance all the N in I, II, and III is \[s{{p}^{2}},\]hybridised and hence statement (4) is CORRECT
Statement (3) is true. Statement (4): Due to resonance all the N in I, II, and III is \[s{{p}^{2}},\]hybridised and hence statement (4) is CORRECT
You need to login to perform this action.
You will be redirected in
3 sec
