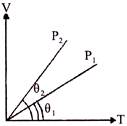
A) \[{{P}_{2}}>{{P}_{1}}\]
B) \[{{P}_{2}}<{{P}_{1}}\]
C) Cannot be predicted
D) \[{{P}_{2}}={{P}_{1}}\]
Correct Answer: B
Solution :
\[{{P}_{1}}>{{P}_{2}}\]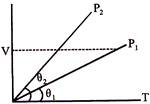 As V = constant \[\Rightarrow \,P\propto T\] Hence from \[V-T\] graph \[{{P}_{1}}>{{P}_{2}}\]
As V = constant \[\Rightarrow \,P\propto T\] Hence from \[V-T\] graph \[{{P}_{1}}>{{P}_{2}}\]
You need to login to perform this action.
You will be redirected in
3 sec
