A)
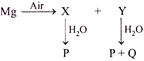
X Y P Q \[MgO\] \[Mg{{(OH)}_{2}}\] \[Mg{{(OH)}_{2}}\] \[{{N}_{2}}\]
B)
\[MgO\] \[M{{g}_{3}}{{N}_{2}}\] \[Mg{{(OH)}_{2}}\] \[N{{H}_{3}}\]
C)
\[MgO\] \[M{{g}_{3}}{{N}_{2}}\] \[Mg{{(OH)}_{2}}\] \[{{N}_{2}}\]
D)
\[MgO\] \[MgC{{O}_{3}}\] \[Mg{{(OH)}_{2}}\] \[C{{O}_{2}}\]
Correct Answer: B
Solution :
Magnesium reacts with air to form oxide and nitride. On reaction with water the oxide gives hydroxide and nitride gives hydroxide and ammonia. \[2Mg+{{O}_{2}}\to \underset{(X)}{\mathop{2MgO}}\,\] \[3Mg+{{N}_{2}}\to \underset{(Y)}{\mathop{M{{g}_{3}}{{N}_{2}}}}\,\] \[MgO+{{H}_{2}}O\to \underset{(P)}{\mathop{Mg{{(OH)}_{2}}}}\,\] \[M{{g}_{3}}{{N}_{2}}+{{H}_{2}}O\to \underset{(P)}{\mathop{3Mg{{(OH)}_{2}}}}\,+\underset{(Q)}{\mathop{2N{{H}_{3}}}}\,\]You need to login to perform this action.
You will be redirected in
3 sec
