A) \[-1\]
B) \[-2\]
C) 0
D) +1
Correct Answer: C
Solution :
[c] \[{{t}_{1/2}}=\frac{0.693}{k}\] \[log\text{ }{{t}_{1}}/2=log0.693-logk=constant\]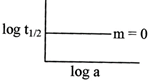
You need to login to perform this action.
You will be redirected in
3 sec
