A) X - \[N{{a}_{2}}S{{O}_{3}}\] Y - \[Cu{{S}_{2}}{{O}_{3}}\] Z - \[N{{a}_{2}}[C{{u}_{5}}{{({{S}_{2}}{{O}_{3}})}_{6}}]\]
B) X - \[N{{a}_{2}}{{S}_{4}}{{O}_{6}}\] Y - \[C{{u}_{2}}({{S}_{2}}{{O}_{3}})\] Z - \[N{{a}_{4}}[C{{u}_{6}}{{({{S}_{2}}{{O}_{3}})}_{5}}]\]
C) X - \[N{{a}_{2}}S{{O}_{4}}\] Y - \[Cu{{S}_{4}}{{O}_{6}}\] Z - \[N{{a}_{3}}[C{{u}_{5}}{{({{S}_{2}}{{O}_{3}})}_{4}}]\]
D) X - \[NaHS{{O}_{4}}\] Y - \[C{{u}_{2}}({{S}_{4}}{{O}_{6}})\] Z - \[Na[C{{u}_{3}}{{({{S}_{2}}{{O}_{3}})}_{2}}]\]
Correct Answer: B
Solution :
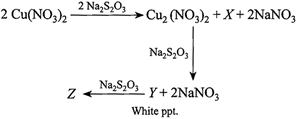
[b] (i) \[2Cu{{(N{{O}_{3}})}_{2}}+2N{{a}_{2}}{{S}_{2}}{{O}_{3}}\xrightarrow{{}}C{{u}_{2}}{{(N{{O}_{3}})}_{2}}+2NaN{{O}_{3}}+\underset{(X)}{\mathop{N{{a}_{2}}{{S}_{4}}{{O}_{6}}}}\,\](ii)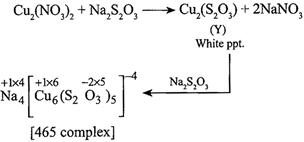
| 4 | 6 | 5 |
| \[N{{a}^{+1}}\] | \[C{{u}^{+1}}\] | \[{{S}_{2}}{{O}_{3}}^{-2}\] |
You need to login to perform this action.
You will be redirected in
3 sec
