A)
![]()
B)
![]()
C)
![]()
D)
![]()
Correct Answer: B
Solution :
\[\mu =\,\,\sqrt{n(n+2)}\] \[3.83 = \sqrt{n\left( n+2 \right)}\] on solving \[\operatorname{n} = 3\] as per magnetic moment, it has three impaired electron. \[{{\operatorname{Cr}}^{3}}^{+}\] will have configuration as \[\operatorname{Cr} :\,\,\,1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{4}}4{{s}^{2}}\] \[\operatorname{C}{{r}^{3+}}:\,\,1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{3}}\]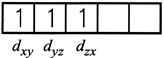
You need to login to perform this action.
You will be redirected in
3 sec
