A)
B)
C)
D)
Correct Answer: B
Solution :
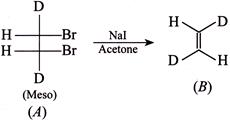
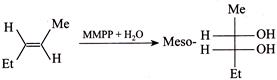
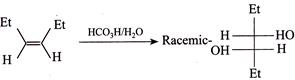
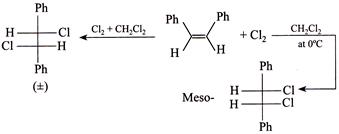
[b] Rule: (With two same group) meso-compound + Anti-elimination of \[B{{r}_{2}}\to \]trans-Alkene. (ii) \[(\pm )\]Compound + Anti-elimination of \[B{{r}_{2}}\to \]cis-alkene. According to the rule (i) trans-Compound (with two different groups) +Anti-addition of two \[(OH)\] groups \[\to (\pm )\] or racemic compound. So the answer is wrong. cis-Compound (with two same groups) + Anti-addition of two \[(OH)\] groups \[\to (\pm )\] or racemic compound. It is the correct answer. Addition of \[C{{l}_{2}}\] in the presence of \[C{{H}_{2}}C{{l}_{2}}\]at \[0{}^\circ C\]is cis-addition; whereas at \[-9{}^\circ C,\] it is trans-addition, e.g.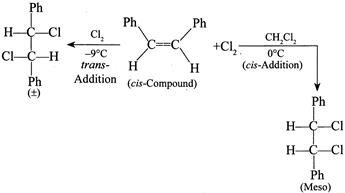
You need to login to perform this action.
You will be redirected in
3 sec
