| Consider the acidity of the carboxylic acids |
| (i)\[PhCOOH\] |
| (ii)\[o-N{{O}_{2}}{{C}_{6}}{{H}_{4}}COOH\] |
| (iii)\[p-N{{O}_{2}}{{C}_{6}}{{H}_{4}}COOH\] |
| (iv)\[m-N{{O}_{2}}{{C}_{6}}{{H}_{4}}COOH\] |
| Which of the following order is correct? |
A) \[i>ii>iii>iv\]
B) \[ii>iv>iii>i\]
C) \[ii>iv>i>iii\]
D) \[ii>iii>iv>i\]
Correct Answer: D
Solution :
[d]: Order of acidity is: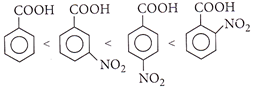 E.W.G. increases the acidity of benzoic acid, o-isomer will have higher acidity than corresponding m-and p-isomers due to ortho-effect. In p-nitrobenzoic acid, both -R effect and -I effect of the nitro group increase the acidity while in m-nitrobenzoic acid, only the weaker -I effect increases the acidity. Therefore the correct order of acidity is it ii>iii>iv>i.
E.W.G. increases the acidity of benzoic acid, o-isomer will have higher acidity than corresponding m-and p-isomers due to ortho-effect. In p-nitrobenzoic acid, both -R effect and -I effect of the nitro group increase the acidity while in m-nitrobenzoic acid, only the weaker -I effect increases the acidity. Therefore the correct order of acidity is it ii>iii>iv>i.
You need to login to perform this action.
You will be redirected in
3 sec
