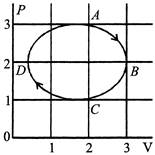
A) the process during the path \[\operatorname{A}\,\to B\] is isothermal
B) heat flows out of the gas during the path \[\operatorname{B}\,\to C\,\to D\]
C) work done during the path \[\operatorname{A} \to B\to C\] is zero
D) negative work is done by the gas in the cycle ABCDA
Correct Answer: B
Solution :
[a] Process is not isothermal [b] Volume decreases and temperature decreases \[\Delta U=negative,\] So, \[\Delta Q= negative\] [c] Work done in process \[A\to B\,\to C\] is positve [d] Cycle is clockwise, so work done by the gas is positiveYou need to login to perform this action.
You will be redirected in
3 sec
