A) \[{{p}_{x}},{{p}_{x}}\]
B) \[{{p}_{x}},{{d}_{xz}}\]
C) \[{{d}_{xz}},{{d}_{xz}}\]
D) \[{{p}_{x}},{{d}_{{{x}^{2}}-{{y}^{2}}}}\]
Correct Answer: D
Solution :
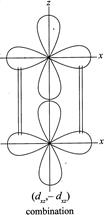
[d] (i) \[({{p}_{x}},{{p}_{x}}),\,({{p}_{x}},{{d}_{xz}}),({{d}_{xz}},{{d}_{xz}})\] (ii) \[({{p}_{y}},{{p}_{y}}),({{p}_{y}},{{d}_{yz}}),({{d}_{yz}},{{d}_{yz}})\] The figures below indicate the combination in xz projection.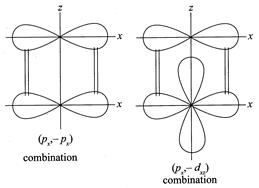
You need to login to perform this action.
You will be redirected in
3 sec
