A) \[{{Z}_{11}}(A)=32\,\,{{Z}_{11}}(B)\]
B) \[32\,{{Z}_{11}}(A)=\,\,{{Z}_{11}}(B)\]
C) \[{{Z}_{11}}(A)=\,16\,{{Z}_{11}}(B)\]
D) \[16\,{{Z}_{11}}(A)=\,{{Z}_{11}}(B)\]
Correct Answer: A
Solution :
[a] Since \[{{Z}_{1}}=\frac{{{\mu }_{av}}}{{{\lambda }_{A}}},\]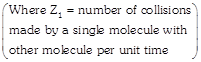 \[{{Z}_{1A}}=\frac{{{\mu }_{av}}(A)}{{{\lambda }_{A}}}\] and \[{{Z}_{1B}}=\frac{{{\mu }_{av(A)}}}{{{\lambda }_{A}}}\] \[\frac{{{\mu }_{av}}(A)}{{{\mu }_{av}}(B)}\,\,\,\sqrt{\frac{{{T}_{A}}}{{{M}_{A}}}\times \frac{{{M}_{B}}}{{{T}_{B}}}}=\sqrt{\frac{400}{2}\times \frac{16}{800}}=2\] \[\frac{{{\lambda }_{A}}}{{{\lambda }_{B}}}=\frac{1}{2}\] (Given) \[\frac{{{Z}_{1}}_{A}}{{{Z}_{1}}_{B}}=\left( \frac{{{\mu }_{av(A)}}}{{{\mu }_{av(B)}}} \right)\left( \frac{{{\lambda }_{B}}}{{{\lambda }_{A}}} \right)=2\times 2=4\] To calculate \[\left( N_{A}^{*}/N_{B}^{*} \right):\] Let \[{{n}_{A}}\] and \[{{n}_{B}}\] are the amount of \[{{H}_{2}}\] and \[C{{H}_{4}}\] \[{{n}_{A}}=\frac{m}{2},\] \[{{n}_{B}}=\frac{m}{16},\]
\[{{Z}_{1A}}=\frac{{{\mu }_{av}}(A)}{{{\lambda }_{A}}}\] and \[{{Z}_{1B}}=\frac{{{\mu }_{av(A)}}}{{{\lambda }_{A}}}\] \[\frac{{{\mu }_{av}}(A)}{{{\mu }_{av}}(B)}\,\,\,\sqrt{\frac{{{T}_{A}}}{{{M}_{A}}}\times \frac{{{M}_{B}}}{{{T}_{B}}}}=\sqrt{\frac{400}{2}\times \frac{16}{800}}=2\] \[\frac{{{\lambda }_{A}}}{{{\lambda }_{B}}}=\frac{1}{2}\] (Given) \[\frac{{{Z}_{1}}_{A}}{{{Z}_{1}}_{B}}=\left( \frac{{{\mu }_{av(A)}}}{{{\mu }_{av(B)}}} \right)\left( \frac{{{\lambda }_{B}}}{{{\lambda }_{A}}} \right)=2\times 2=4\] To calculate \[\left( N_{A}^{*}/N_{B}^{*} \right):\] Let \[{{n}_{A}}\] and \[{{n}_{B}}\] are the amount of \[{{H}_{2}}\] and \[C{{H}_{4}}\] \[{{n}_{A}}=\frac{m}{2},\] \[{{n}_{B}}=\frac{m}{16},\] 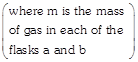 \[\frac{{{n}_{A}}}{{{n}_{B}}}=8\] The number of molecules in flasks A and B are: \[N_{A}^{*}=\frac{m}{2}{{N}_{A}},\] \[N_{B}^{*}=\frac{m}{16}{{N}_{A}}\]
\[\frac{{{n}_{A}}}{{{n}_{B}}}=8\] The number of molecules in flasks A and B are: \[N_{A}^{*}=\frac{m}{2}{{N}_{A}},\] \[N_{B}^{*}=\frac{m}{16}{{N}_{A}}\] You need to login to perform this action.
You will be redirected in
3 sec
