A) \[1.33\]
B) \[2.66\]
C) \[2.00\]
D) \[3.00\]
Correct Answer: A
Solution :
[a]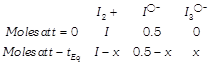 Excess \[AgN{{O}_{3}}\] gave \[0.25\text{ }mol\]of yellow precipitate.
Excess \[AgN{{O}_{3}}\] gave \[0.25\text{ }mol\]of yellow precipitate. 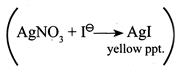 \[\Rightarrow \,\,\,\,\,\,0.5-x=0.25\] \[\Rightarrow \,\,\,\,\,\,x=0.25\]
\[\Rightarrow \,\,\,\,\,\,0.5-x=0.25\] \[\Rightarrow \,\,\,\,\,\,x=0.25\] 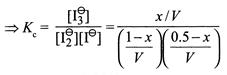 \[=\frac{0.25/1}{\left( \frac{0.75}{1} \right)\left( \frac{0.25}{1} \right)}=1.33\,(V=1.0L)\]
\[=\frac{0.25/1}{\left( \frac{0.75}{1} \right)\left( \frac{0.25}{1} \right)}=1.33\,(V=1.0L)\]
You need to login to perform this action.
You will be redirected in
3 sec
