| A redox reaction is shown in the diagrams. Identify the reaction. |
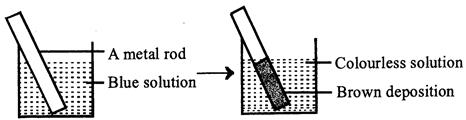 |
A) \[Zn(s)+C{{u}^{2+}}(aq)\xrightarrow{{}}Z{{n}^{2+}}(aq)+Cu(s)\]
B) \[Cu(s)+A{{g}^{+}}(aq)\xrightarrow{{}}C{{u}^{2+}}(aq)+2Ag(s)\]
C) \[2Ag(s)+C{{u}^{2+}}(aq)\xrightarrow{{}}2A{{g}^{+}}(aq)+Cu(s)\]
D) \[Cu(s)+Z{{n}^{2+}}(aq)\xrightarrow{{}}C{{u}^{2+}}(aq)+Zn(s)\]
Correct Answer: A
Solution :
Zinc rod dipped in blue copper sulphate solution is oxidised to \[Z{{n}^{2+}}\] and \[C{{u}^{2+}}\] are reduced to \[Cu\]and get deposited on zinc rod.You need to login to perform this action.
You will be redirected in
3 sec
