| I. \[K[Co{{(N{{H}_{3}})}_{2}}{{(N{{O}_{2}})}_{4}}]\] |
| II. \[[Cr{{(N{{H}_{3}})}_{3}}{{(N{{O}_{2}})}_{3}}]\] |
| III. \[{{[Cr{{(N{{H}_{3}})}_{5}}(N{{O}_{2}})]}_{3}}\,{{[Co{{(N{{O}_{2}})}_{6}}]}_{2}}\] |
| IV. \[Mg[Co(N{{H}_{3}})\,{{(N{{O}_{2}})}_{5}}]\] |
A) \[II<I<III<IV\]
B) \[II<I<IV<III\]
C) \[III<IV<II<I\]
D) \[IV<III<I<II\]
Correct Answer: B
Solution :
| [b] |
(I) 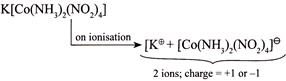 |
| II, \[[Cr{{(N{{H}_{3}})}_{3}}{{(N{{O}_{2}})}_{3}}]\]being non-electrolyte, does not ionise. |
III. 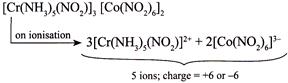 |
IV. 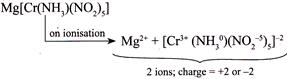 |
| Greater the number of ions and greater the total (positive or negative) charge produced after ionisation, greater is the value of molar conductivity. Hence, increasing order of molar conductivity is \[II<I<IV<III\] |
You need to login to perform this action.
You will be redirected in
3 sec
