Answer:
(a)
(i) It is because there is +ve charge on o- and p-position, therefore,
electrophilic substitution take place at m-position as shown in the following
resonating structures.
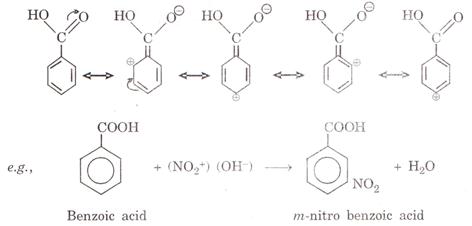 1
(ii)
Carboxylic acids have higher boiling points than alcohols because they operate hydrogen
bonding among their molecules. Hydrogen bonding among acid molecules is far
stronger than among alcohol molecules.
1
(ii)
Carboxylic acids have higher boiling points than alcohols because they operate hydrogen
bonding among their molecules. Hydrogen bonding among acid molecules is far
stronger than among alcohol molecules.
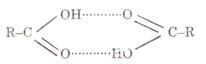 (b)
(i) Propanone to propene:
(b)
(i) Propanone to propene:
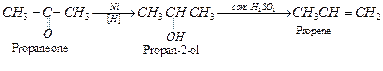 1
(ii)
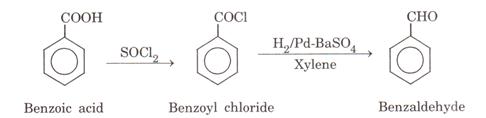
Benzoic acid to benzaldehyde:
1
(ii)
Benzoic acid to benzaldehyde:
 1
(iii)
Ethanol to 3-hydroxybutanal:
1
(iii)
Ethanol to 3-hydroxybutanal:
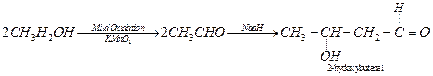 1
1
You need to login to perform this action.
You will be redirected in
3 sec
