
| Experiment |
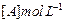
|
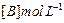
|
Initial rate of formation of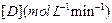
|
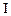

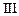
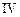
|
0.1 0.3 0.3 0.4 | 0.1 0.2 0.4 0.1 |
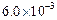
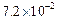
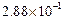
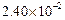
|
Answer:
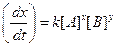
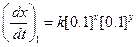
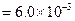 ?(i)
?(i)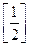
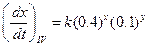
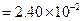 ?(ii)
?(ii) 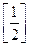 On comparison, we get
On comparison, we get
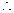
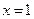 [1]
[1]
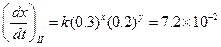
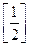
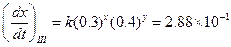
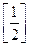 Then,
Then,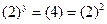 Thus,
Thus,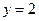 Thus, rate law is
Thus, rate law is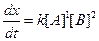 From any experiment
From any experiment
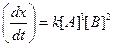
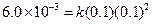
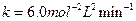 [1]
Or
(a) Pseudo first order reaction Certain reactions which are biomolecular follow first order kinetics as concentration of one of the reactants is very high and does not change appreciably and also slow step (rate-determing step) involves only one reactant. Such types of reactions are called pseudo first order reactions.
Examples
(i) Hydrolysis of ester by acid
[1]
Or
(a) Pseudo first order reaction Certain reactions which are biomolecular follow first order kinetics as concentration of one of the reactants is very high and does not change appreciably and also slow step (rate-determing step) involves only one reactant. Such types of reactions are called pseudo first order reactions.
Examples
(i) Hydrolysis of ester by acid
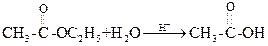
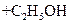 Water is present in large excess and its concentration does not change and
Water is present in large excess and its concentration does not change and
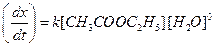
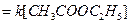 [1]
(ii)
[1]
(ii)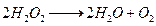
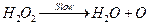
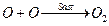 [1]
By slow step
[1]
By slow step
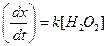 (b)
(b) 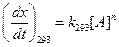
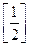
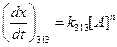
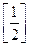 Given,
Given, 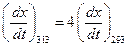
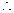
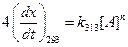 Thus,
Thus, 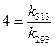 [1]
For energy of activation
[1]
For energy of activation 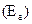
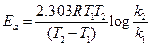
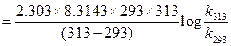

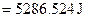
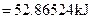 [1]
[1]
You need to login to perform this action.
You will be redirected in
3 sec
